Anionic Bitumen Emulsion
General Description of Anionic Bitumen Emulsion
Chemical surface-active agents, serving as emulsifiers, are classified by the electrochemical charge that is attained when they dissociate in a water solution. In the case of anionic emulsions, the chemical charge is negative. The chemical type and quantity of surface-active agents used in the manufacturing process govern the process in which the resulting asphalt emulsion can be used.
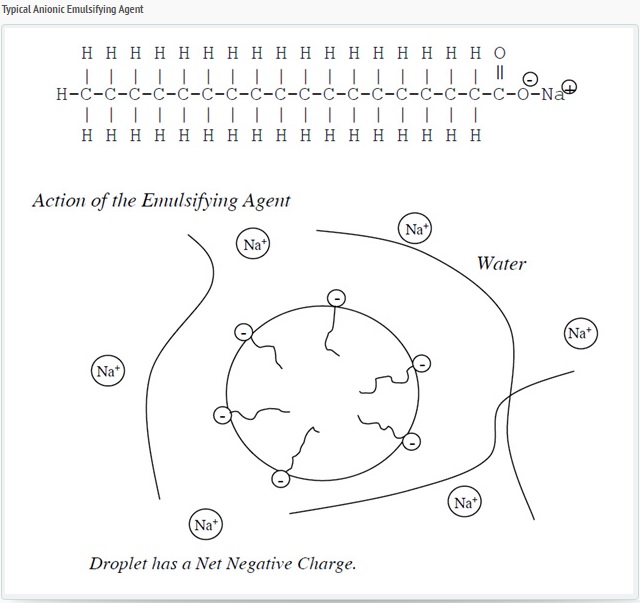
Anionic Bitumen emulsion in which the droplets of bitumen carry a negative charge. The anionic bitumen emulsion is derived from the migration of particles of bitumen under an electric field. The droplets migrate toward the anode (positive electrode), and hence the emulsion is called anionic. In an anionic emulsion, there are “billions and billions” of bitumen droplets with the emulsifying agent at the water bitumen interface. The tail portion of the emulsifying agent aligns itself in the bitumen while the positive portion of the head floats around in the water leaving the rest of the head negatively charged and at the surface of the droplet. This imparts a negative charge to all the droplets. Since negatives repel each other, all the droplets repel each other and remain as distinct bitumen drops in suspension.
In the early days of asphalt emulsion production, materials such as ox-blood, clays, and soaps were used as emulsifying agents. As emulsion demand increased, more efficient emulsifying agents were found. Many chemical emulsifiers are now commercially available.
The most common anionic emulsifiers are acids, which are wood-product derivatives such as tall oils, rosins, and lignin’s. Anionic emulsifiers are saponified (turned into soap) by reacting with sodium hydroxide or potassium hydroxide.
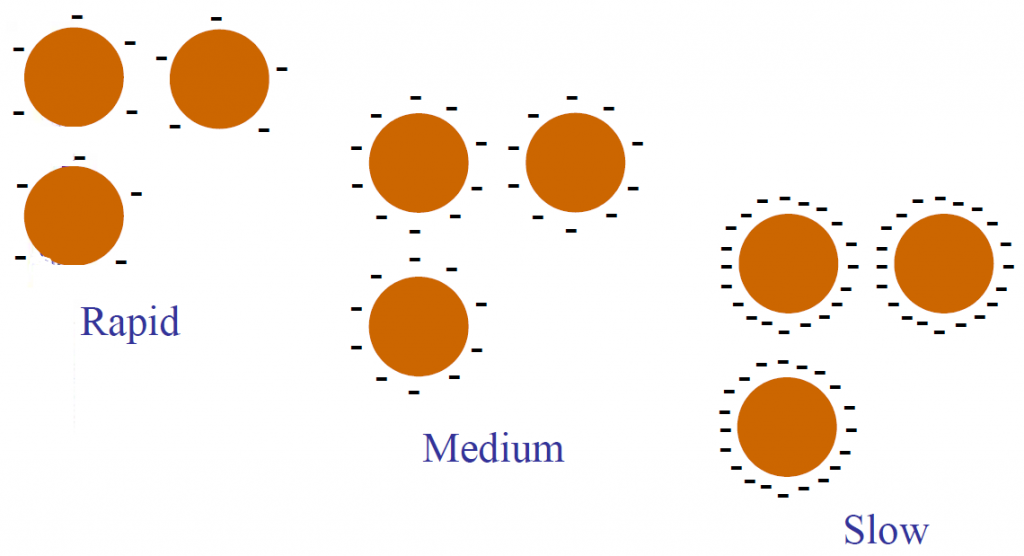
Emulsions are further classified on the basis of how quickly the asphalt droplets will coalesce; (i.e., revert to asphalt cement). The terms RS, MS, SS and QS have been adopted to simplify and standardize this classification. They are relative terms only and mean rapid-setting, medium-setting, slow-setting and quick-setting. The tendency to coalesce is closely related to the speed with which an emulsion will become unstable and break after contacting the surface of an aggregate. An RS emulsion has little or no ability to mix with an aggregate, an NIS emulsion is expected to mix with coarse but not fine aggregate, and SS and QS emulsions are designed to mix with fine aggregate, with the QS expected to break more quickly than the SS.
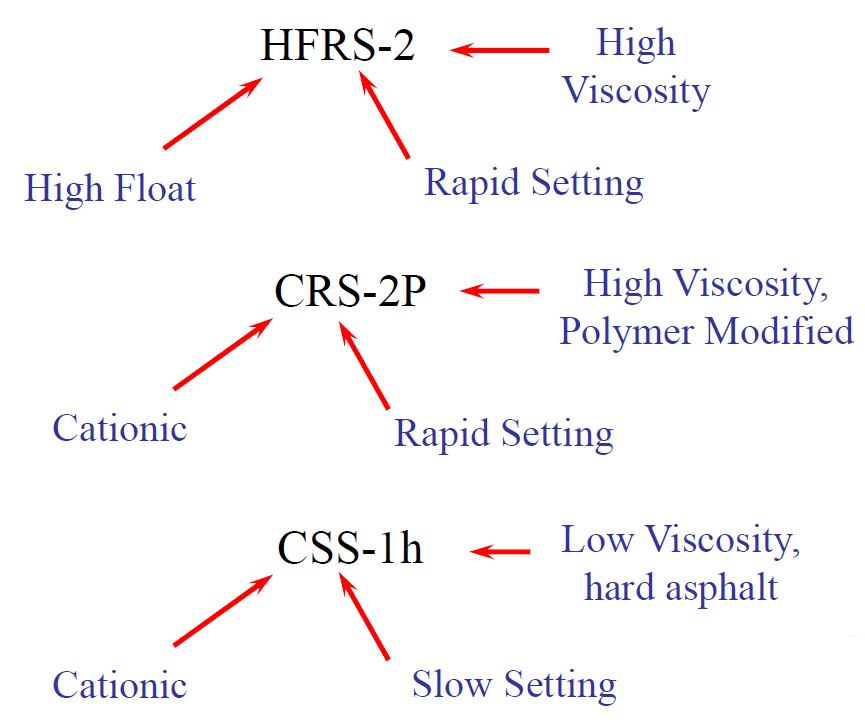
Emulsions are further identified by a series of numbers and letters related to viscosity of the emulsions and hardness of the base asphalt cements. The letter “C” in front of the emulsion type denotes cationic. The absence of the “C” denotes anionic in American Society for Testing and Materials (ASTM) and American Association of State Highway and Transportation Officials (AASHTO) specifications. For example, RS-1 is anionic and CRS-I is cationic.
The numbers in the classification indicate the relative viscosity of the emulsion. For example, an MS-2 is more viscous than an MS-1. The “h” that follows certain grades simply means that harder base asphalt is used. An “s” means that softer base asphalt is used.
The “HF” preceding some of the anionic grades indicates high-float, as measured by the float test. High float emulsions have a gel quality, imparted by the addition of certain chemicals, that permits a thicker asphalt film on the aggregate particles and prevents the drain off of asphalt from the aggregate. These grades are used primarily for cold and hot plant mixes, seal coats, and road mixes.
RAHA Group has been equipped by the DenimoTech full automatic inline emulsion plant which is able to produce different bitumen emulsion grades with superior quality. Some of our anionic emulsions that are classified according to ASTM and EN standards are here
Breaking Characteristics of Anionic Bitumen Emulsion
Emulsions exist for ease of application. After application the water to should evaporate and leave the asphalt cement. In a surface treatment, after emulsion and aggregate have been applied to the road surface, the emulsion should “break” leaving the asphalt cement holding the aggregate. At that point traffic may be allowed on the surface without loss of aggregate. The type of emulsion used has a large effect on the speed of the “break” of an emulsion.
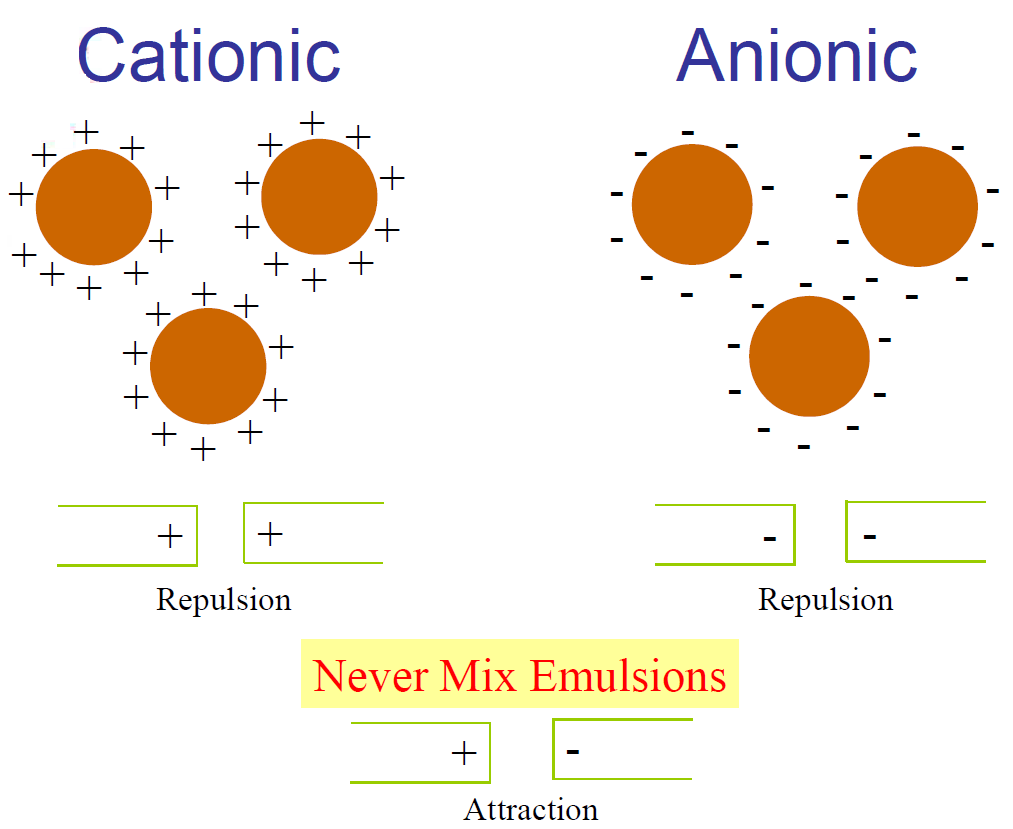
Almost all surfaces have a net negative charge. The strength or intensity of this negative charge may be different from material to material. Because of this phenomenon, anionic and cationic emulsions break in different ways.
In an application of anionic emulsion, negatively charged drops of asphalt are applied to a negatively charged surface. All components repel each other. The only way the emulsion can break is through the loss of water by evaporation. As more and more water is lost through evaporation, the particles are forced closer and closer together until they can no longer be separated by a film of water. At this point, droplets coalesce into larger and larger drops and ultimately a sheet of asphalt on the road. A depiction of the application is shown below:
Anionic Emulsifier Structure
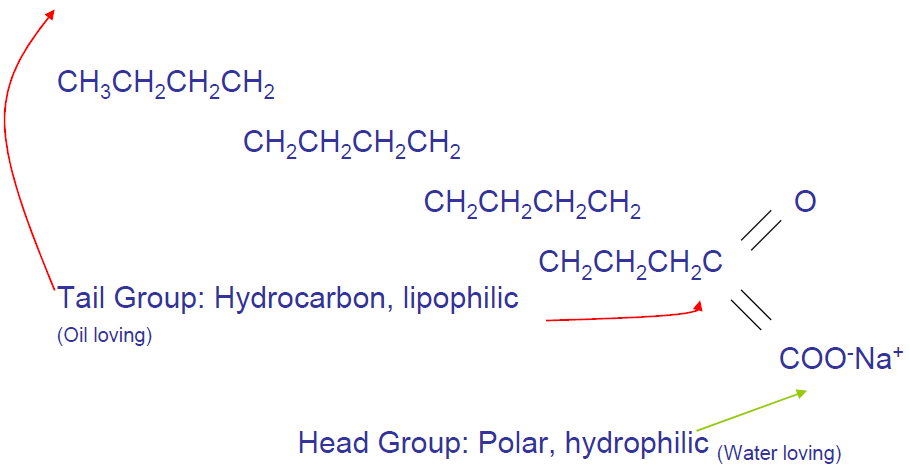
Chemical Set of Bitumen Emulsion
Naming the Emulsions
- RS= Rapid Set
- SS= Slow Set
- QS= Quick Set
- MS= Medium Set
- HFRS= High Float Rapid Set
- C= Cationic
- AE= Anionic