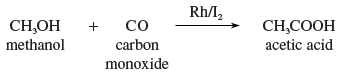Description of Acetic acid:
Acetic acid (CH3COOH), also called ethanoic acid, the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar; a salt, ester, or acylal of acetic acid is called acetate.
Industrially, acetic acid is used in the preparation of metal acetates, used in some printing processes; vinyl acetate, employed in the production of plastics; cellulose acetate, used in making photographic films and textiles; and volatile organic esters (such as ethyl and butyl acetates), widely used as solvents for resins, paints, and lacquers. Biologically, acetic acid is an important metabolic intermediate, and it occurs naturally in body fluids and in plant juices.
Acetic acid, systematically named ethanoic acid, is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2).
When undiluted, it is sometimes called glacial acetic acid. Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Acetic acid has a distinctive sour taste and pungent smell.
In addition to household vinegar, it is mainly produced as a precursor to polyvinyl acetate and cellulose acetate. It is classified as a weak acid since it only partially dissociates in solution, but concentrated acetic acid is corrosive and can damage skin.
Acetic acid has been prepared on an industrial scale by air oxidation of acetaldehyde, by oxidation of ethanol (ethyl alcohol), and by oxidation of butane and butene.
Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117.9 °C [244.2 °F]; melting point 16.6 °C [61.9 °F]) that is completely miscible with water.
Acetic acid, CH3COOH, is a weak acid with many scientific and research applications, including luminescence and mass spectrometry. This organic compound has antibacterial properties and may be involved in lipid solubility and fatty acid accumulation on cell membranes.
Sometimes known as ethanoic acid, acetic acid makes up the largest part of vinegar, after water.
Uses of Acetic acid:
Acetic acid is a chemical reagent for the production of chemical compounds. The largest single use of acetic acid is in the production of vinyl acetate monomer, closely followed by acetic anhydride and ester production. The volume of acetic acid used in vinegar is comparatively small.
There are many uses of acid. So, in addition to being treated just as a food preservative (vinegar), the acid is used in many areas and instances. Some top and important uses include:
- Industrial Use
- Medicinal Uses
- Household
- Food Industry
Industrial Use:
Acetic acid is used in many industrial processes for the production of substrates and it is often used as a chemical reagent for the production of a number of chemical compounds like acetic anhydride, ester, vinyl acetate monomer, vinegar, and many other polymeric materials. It is also used to purify organic compounds as it can be used as a solvent for recrystallization.
Medical Use:
Acetic acid has a lot of uses in the medical field. The most important uses here is that it can be used as an antiseptic against pseudomonas, enterococci, streptococci, staphylococci and others. It is also used in cervical cancer screening and for the treatment of infections. Further it is used as an agent to lyse red blood cells before white blood cells are examined. Vinegar has also been said to reduce high concentrations of blood sugar.
Food Industry:
In the food industry, acetic acid finds its use most commonly in commercial pickling operations, and in condiments like mayonnaise, mustard and ketchup. It is also used for seasoning various food items like salads etc. Additionally, vinegar can react with alkaline ingredients like baking soda and when that happens it produces a gas which helps to make baked goods become more puffy.
Household Uses:
Acetic acid which is a dilute solution is used extensively as vinegar. And as we are familiar, vinegar is widely used for cleaning, laundry, cooking, and many other household uses. Farmers usually spray acetic acid on livestock silage to counter bacterial and fungal growth.
Apart from these, acetic acid is used for the manufacture of inks and dyes and it is also used in making perfumes. It is also involved in manufacturing of rubber and plastic industries.
Use as solvent:
Glacial acetic acid is an excellent polar protic solvent. It is frequently used as a solvent for recrystallization to purify organic compounds. Acetic acid is used as a solvent in the production of terephthalic acid (TPA), the raw material for polyethylene terephthalate (PET). In 2006, about 20% of acetic acid was used for TPA production.
Acetic acid is often used as a solvent for reactions involving carbocations, such as Friedel-Crafts alkylation. For example, one stage in the commercial manufacture of synthetic camphor involves a Wagner-Meerwein rearrangement of camphene to isobornyl acetate; here acetic acid acts both as a solvent and as a nucleophile to trap the rearranged carbocation.
Specification of Acetic Acid:
| Physical Form | Liquid |
| Packaging | HDPE plastic bottle |
| Vapor Density | 2.1 |
| pH | 2.5 |
| Boiling Point | 117°C |
| Molecular Formula | C2H4O2 |
| Formula Weight | 60.05g/mol |
| Nickel (Ni) | ≤0.1ppm |
| Vapor Pressure | 1.52kPa at 20°C |
| Melting Point | 16°C |
| Color | Colorless |