caustic soda production process
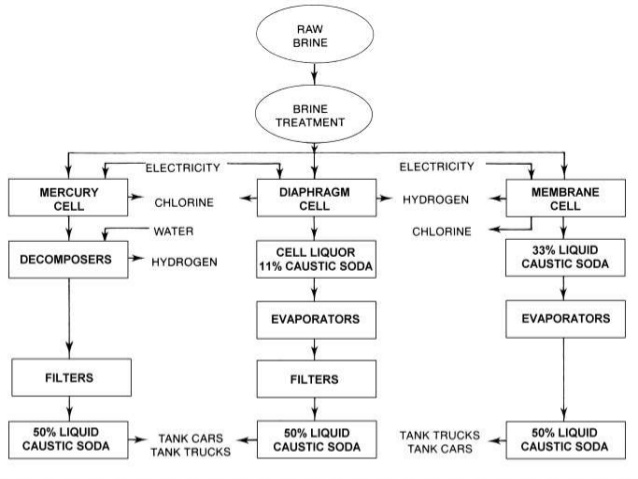
Caustic soda production
Nearly all caustic soda is generated by the electrolysis of sodium chloride solution using one of three cell types: mercury, diaphragm and membrane cells. The electrolysis process produces 2.25 tonnes of 50% caustic soda with each tonne of chlorine.
The primary raw material is common salt, usually in the form of underground deposits which are brought to the surface as a solution in a pumped high pressure water supply. The sodium chloride solution is often called brine.
Traditionally, electrolysis has been carried out by the mercury amalgam or diaphragm cell processes but the ion exchange membrane cell is taking a growing share on environmental and economic grounds. The main production route in the US is via the diaphragm cell while the mercury and membrane cells are more dominant in Europe. There is environmental pressure on the older mercury route, with some bodies recommending a phase-out by 2010.
In the mercury cell, sodium is discharged in the form of a mercury sodium amalgam and chloride ions as chlorine. The amalgam flows to a totally separate compartment where it reacts with water to yield sodium hydroxide solution and hydrogen gas.
The diaphragm cell, usually manufactured of asbestos, allows a flow of brine from the anode to cathode but separates the chlorine and hydrogen gas spaces. As hydrogen ions are discharged, hydroxide ions accumulate in the cathode compartment with the aqueous sodium ions to produce sodium hydroxide. Back migration of the hydroxide ions from the cathode to anode is prevented by the velocity of the liquid flow from one compartment to the other. The chlorine formed at the anodes rises through the brine into a space formed by the cell’s cover.
In the membrane process, the ion exchange membrane acts as a barrier to all gas and liquid flows, and only allows the passage of sodium ions between compartments. The sodium ions pass in hydrated form to produce sodium hydroxide in the cathode where hydrogen is given off. Chlorine gas is liberated at the anode. The membrane is a copolymer of tetrafluoroethylene or a similar fluorinated monomer.
Mercury cells are cheaper to operate than diaphragm cells when electricity costs are low and produce product at the required concentration and high purity, but mercury must be removed from the effluent. Diaphragm cells need plenty of thermal energy to concentrate the caustic solutions but can be cheaper than mercury cells when steam costs are low and have relatively cheap construction costs. Use of membrane cells is growing due to lower capital and energy costs and an absence of environmental problems. The product is of high purity but needs further concentration if 50% caustic soda is required. Membranes can handle varying current densities and maximise production at cheap electricity prices.
The lime soda process produces lower grade caustic soda suitable for some applications. Treatment of Trona ore (a mixture of sodium carbonate and sodium bicarbonate) with lime is used to produce sodium hydroxide. One Romanian plant utilises the lime soda process.

