What is Caustic Soda (sodium hydroxide)?
“Caustic soda” is a term that might be unfamiliar to those who haven’t worked with industrial chemicals. However, it is a substance used either as an ingredient or in the process of manufacturing dozens of household products (such as body soap, detergents and drain cleaners). Often referred to by its household name of “lye,” which literally means “wash stuff” in Old English, caustic soda has probably been used since the time of the ancient Babylonians and Egyptians, who used it in saponification (the soap-making process).
Caustic soda is a relatively simple man-made chemical compound. It is comprised of one sodium (Na), one oxygen (O) and one hydrogen (H) atom, which is why it’s also referred to as sodium hydroxide bearing the chemical symbol “NaOH.”
Caustic soda is probably the lesser-known product of the Chloralkali process, which is the electrolysis procedure used to free chlorine (Cl) from the sodium chloride (NaCl) found in salt brine. When chemical manufacturers produce chlorine, they also end up producing a comparable amount of caustic soda, which is in many ways as useful as its chemical counterpart.
It is important to recognize that the terms “caustic soda,” “sodium hydroxide” and “lye” all refer to the same chemical compound of NaOH. These terms are used interchangeably throughout the industry and throughout this guide.
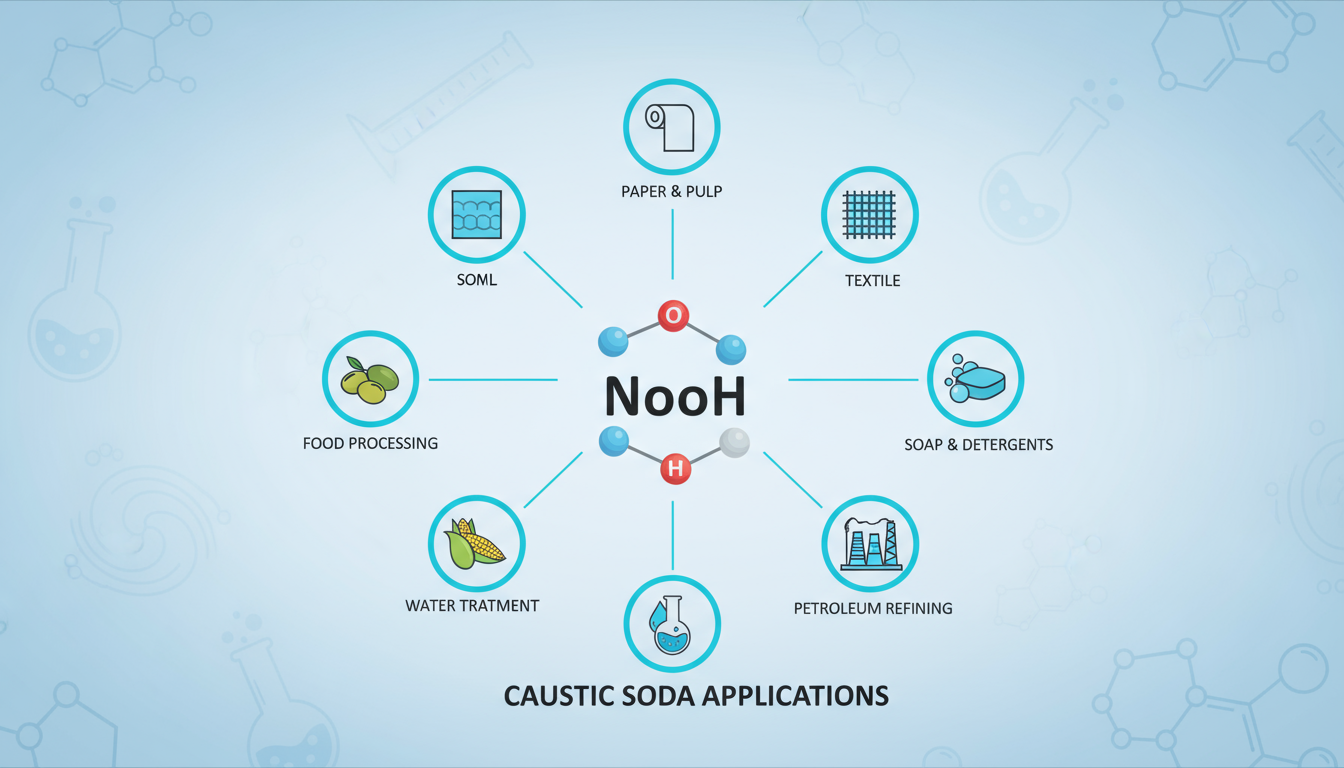
Safety and Application of Caustic Soda in Different Industries
- Textile Industry: Used in mercerization and bleaching processes to improve fabric strength, dye absorption, and brightness.
- Paper & Pulp Industry: Plays a key role in pulping and bleaching, helping to separate lignin from cellulose and produce high-quality paper.
- Soap & Detergent Industry: Essential in saponification, converting fats and oils into soap, and also used in detergent formulations.
- Petroleum & Chemical Industry: Applied in refining petroleum products, removing acidic contaminants, and producing various organic and inorganic chemicals.
- Water Treatment: Neutralizes acidity in water and helps in the removal of heavy metals and impurities.
- Food Industry: Used in regulated amounts for processing foods such as curing olives, making cocoa, and peeling fruits/vegetables.
- Aluminum & Metal Processing: Helps extract aluminum from bauxite ore and is used in cleaning, etching, and surface preparation of metals.
- Pharmaceutical Industry: Supports the production of intermediates and active ingredients in medicines.
- Glass & Ceramics: Contributes to manufacturing processes that require strong alkaline conditions.
Caustic soda flakes are valued for their versatility, purity, and ease of handling, making them indispensable across numerous industries.
Caustic soda is a highly corrosive base or alkaline chemical widely utilized in several different industries. Some common uses include organic chemical production, cosmetic production, soaps & detergent production, paint manufacturing, paper and cellulose, textile bleaching, glass and ceramic manufacturing, food processing, manufacturing rayon, fuel cell production, water treatment, and plumbing (drain cleaners often contain lye).
Application of Caustic soda can cause severe burns to the skin, so it may seem counterintuitive to use lye for making soap. However, sodium hydroxide is one of the few inorganic materials allowed by the USDA in the manufacture of organic soaps. The United States Department of Agriculture (USDA) maintains strict rules over many products grown, raised and produced for human consumption.
When combined with oils or fats in the saponification process, the application of caustic soda compound is transformed during the reaction to make the soap safe to use. While the soap acquires some of the atoms once existing in the caustic soda molecule, they are no longer part of the sodium hydroxide compound: the sodium hydroxide and its corrosive properties are gone.
Application of Caustic Soda as Chemical

At room temperature, caustic soda is a solid, but because it dissolves readily in water, it is often sold and transported as solutions of varying concentrations. When mixed with water or an acid, caustic soda undergoes a strong exothermic reaction that releases heat, which can be harnessed to initiate other chemical processes. In many lye-related reactions—such as soap production—caustic soda contributes its sodium, hydrogen, and oxygen atoms to form new compounds.
In applications where a corrosive substance is required, such as clearing drain blockages, caustic soda effectively dissolves organic matter while leaving PVC pipes intact. Sodium hydroxide also has controlled applications in the food industry, such as in the production of soft drinks, ice cream, and food dyes. Importantly, while the compound itself is highly corrosive, its toxic properties disappear in these controlled processes, similar to the way soap becomes safe for use after saponification.
It is important to note that sodium hydroxide is not safe for direct consumption. Ingesting it can cause severe injury or death. For this reason, food manufacturers enforce strict training protocols for employees handling caustic soda and rigorously test all end products before they reach consumers.
Transporting Caustic Soda
The transportation of hazardous materials, including caustic soda, is regulated by overlapping federal, state, and local laws, as well as international standards. In addition to legal requirements, the chemical properties of sodium hydroxide impose natural restrictions on how it can be packaged and shipped safely.
Caustic soda reacts with certain metals such as aluminum, magnesium, zinc, tin, and chromium, releasing hydrogen gas that can be explosive. Therefore, it must never be stored in containers made of these materials. Stainless steel is the industry standard for drums, tanks, and pipelines since it resists corrosion. For plastics, high-density polyethylene (HDPE) is the preferred choice, as caustic soda can weaken or corrode other types of plastic.
Because caustic soda releases heat when exposed to moisture, the solid form must be kept in airtight containers to prevent contact with water. Paper and cardboard packaging are unsuitable, as they are porous and combustible. Liquid sodium hydroxide can be transported in tanks, drums, or pipelines, following the same strict requirements for container materials as the solid form.
Hazardous Material Classifications and Warnings
In the United States, the Pipeline and Hazardous Materials Safety Administration (PHMSA) regulates the transport of dangerous substances. According to the Federal Motor Carrier Safety Administration (FMCSA), sodium hydroxide is classified as a Class 8 (corrosive) hazardous material. Transport vehicles must display the proper placard, using UN number 1823 for solid caustic soda and 1824 for liquid solutions. These placards follow the black-and-white diamond format, depicting a material dissolving a solid and burning a hand.
Supplier of Caustic Soda Flakes Worldwide:
RAHA Oil Co is a dependable supplier of caustic soda, offering high-quality sodium hydroxide Flakes to meet diverse industrial requirements. With a strong commitment to consistency and safety, RAHA Oil Co ensures its products adhere to both domestic and international standards.
Our efficient logistics and secure packaging mean reliable and timely delivery, making Us a trusted partner.
for more information and inquiry please contact us below:
📱+90 507 246 95 20 / +98 913 288 4959
🏢Head Office: Dubai, Business Bay / Ankara, Turkey
📧salem@rahaoil.com / info@rahaoil.com
🌐https://www.rahaoil.com